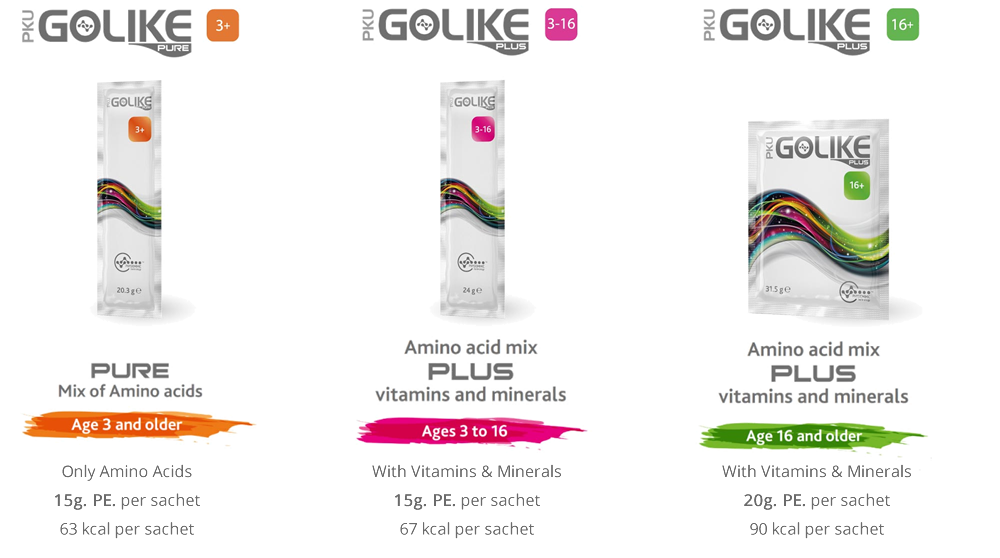
PKU GOLIKE: THE EVOLUTION in PKU NUTRITIONAL MANAGEMENT
Nowadays, the standard of care for phenylketonuria (PKU) is mainly based on two essential pillars: a lifelong low protein diet (that limits Phe intake from foods) and protein substitute administration (to support physiological protein synthesis)1,2.
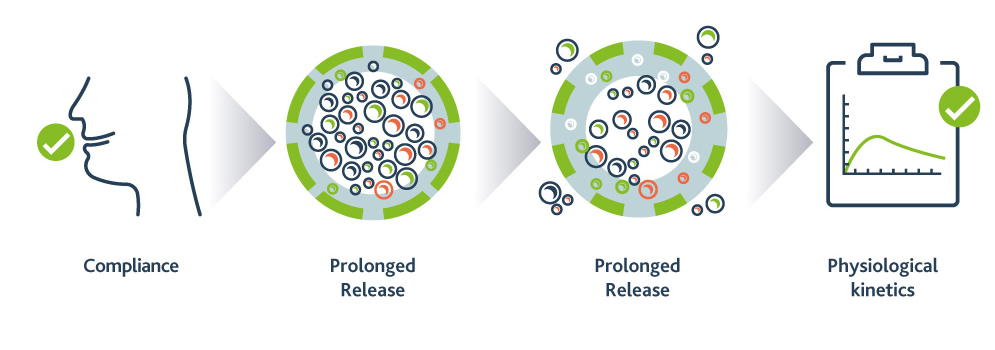
The main objective of the treatment is to maintain Phe levels in the recommended range, and the efficacy of the treatment is strongly influenced by compliance to the prescribed diet. As seen on evidence, compliance becomes increasingly difficult with age due to diverse factors. Bad taste, odour and aftertaste of amino acid-based protein substitutes are still a big issue that generate an important number of adults out of diet3.
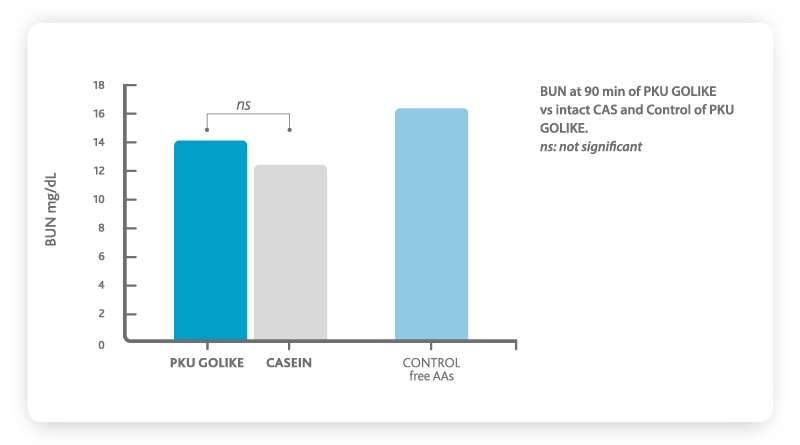
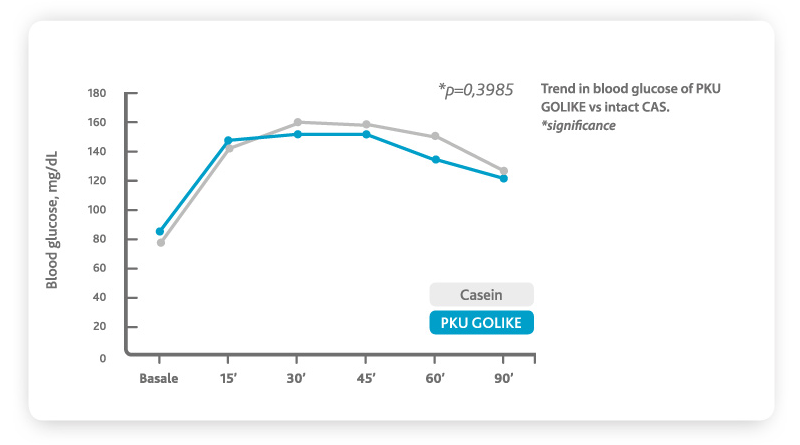
Moreover, scientific evidence indicates that significant sub-optimal health outcomes still exist in compliant PKU patients
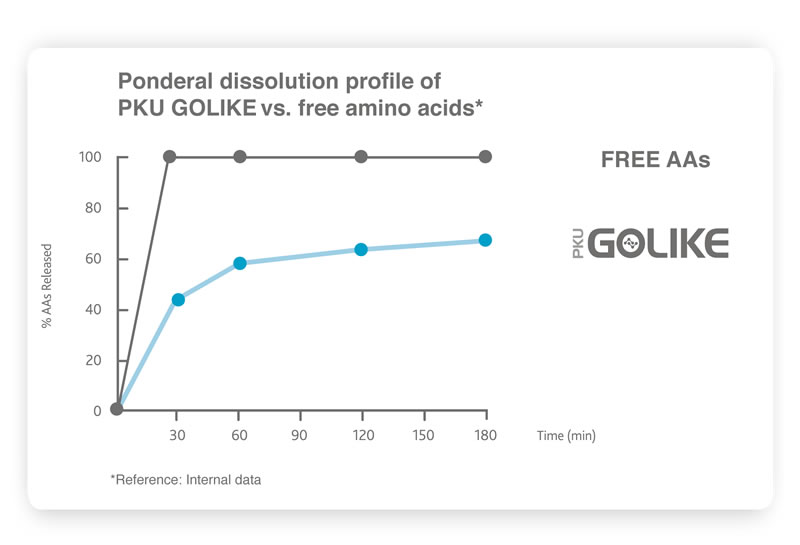
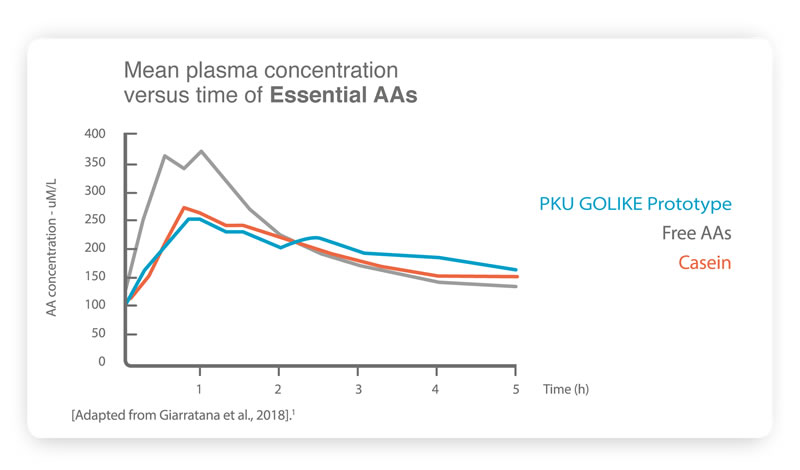
6. This is mainly due to the absorption profile of free amino acids (AA), which is very different from that of intact natural proteins. Free amino acids bypass the digestive phase giving place to plasma levels of amino acids that are higher, peak faster and decrease more quickly
1. Thus, the diverse kinetic profile of free amino acids has an
impact on body metabolism and consequently affects the health of people with PKU
1.
That is where PKU GOLIKE comes in!
PKU GOLIKE is a brand-new food for special medical purposes (FSMP) for a real change in the dietary management of phenylketonuria.
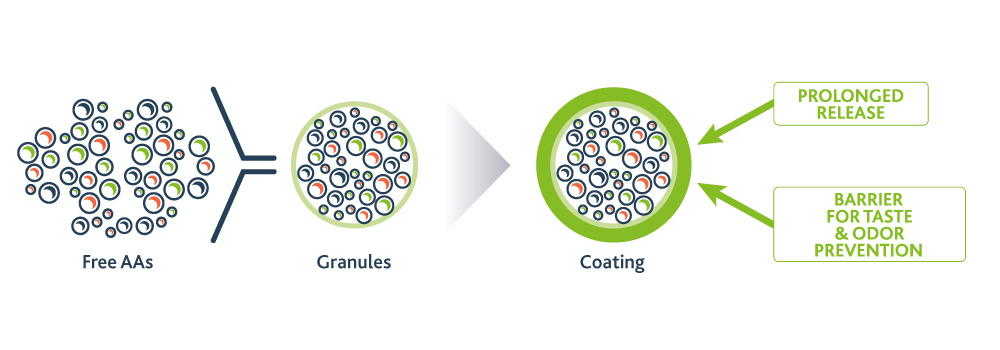
PKU GOLIKE is a Phe-free amino acid mix in granules, characterised by prolonged release and physiological absorption of amino acids, while masking their unpleasant taste and odour, as well as preventing aftertaste.
PKU GOLIKE has been developed with a rigorous pharmaceutical approach, with the ambition of significantly improving health status and quality of life of people living and dealing with PKU.